BACKGROUND
Of any biological system enzymes are the catalyst and they are very efficient as well as specific like that of catalysts. The most important function of an enzyme is to accelerate the reaction rate by million factors as compared to the rate of that reaction when enzymes are not present.
| Telegram | Subscribe |
| Follow | |
| Website | Click here |
According to Michaelis-Menten equation1
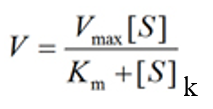
Vo = Initial reaction velocity
Vmax = Maximum velocity
Km = Michaelis constant
[S] = Substrate concentration.
Km is enzyme characteristics and is particular substrate that reflects the affinity of the enzymes for the substrate. It is numerically equal to the concentration of the substrate at which the reaction velocity is same as half value of Vmax.
It is not dependent on enzyme concentration.
Small Km indicates the enzymes high affinity for the substrates low concentration of the substrate is required for saturating enzymes. Large Km is the low affinity of the enzymes for substrate.2
REQUIREMENTS
Chemicals: Enzymes
Starch solution – 2% stock and 0.1-2% working solution.
0.1 HCl
Iodine solution- Prepare it by adding 5 g of potassium iodide to 100 ml of water.
Then this dissolved potassium iodide is added to 1 g of iodide and then allows to completely dissolve.
Amylase solution
Potassium phosphate buffer having pH-7
Apparatus: Spectrophotometer
Glasswares: Test tubes
PROCEDURE
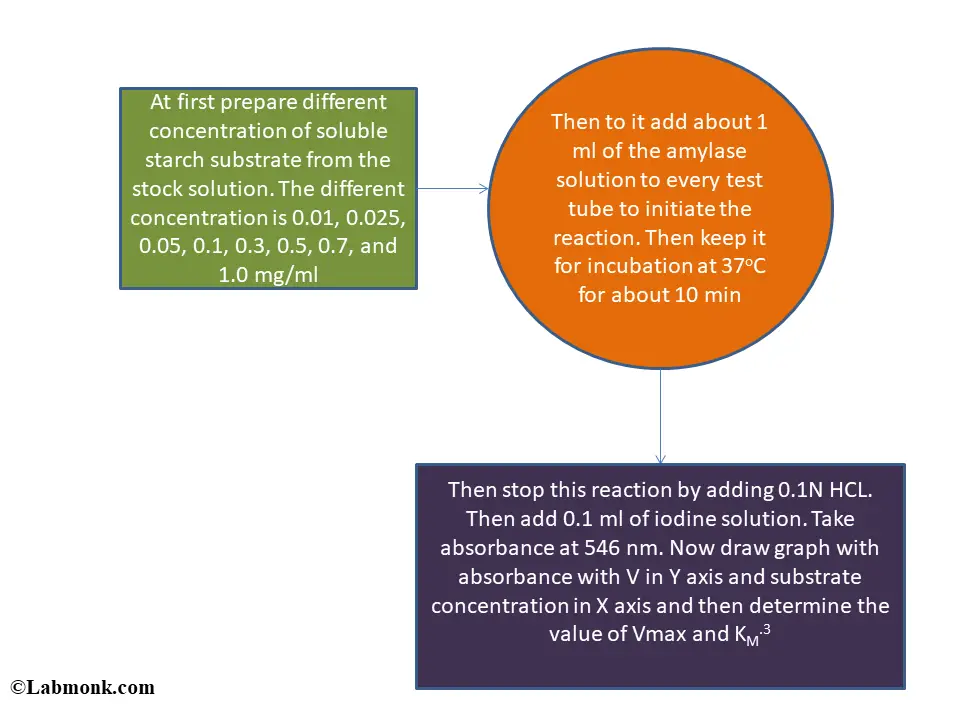
At first prepare different concentration of soluble starch substrate from the stock solution. The different concentration is 0.01, 0.025, 0.05, 0.1, 0.3, 0.5, 0.7, and 1.0 mg/ml.3 Then to it add about 1 ml of the amylase solution to every test tube to initiate the reaction. Then keep it for incubation at 37oC for about 10 min.4,5
Then stop this reaction by adding 0.1N HCl. Then add 0.1 ml of iodine solution. Take absorbance at 546 nm. Now draw graph with absorbance with V in Y axis and substrate concentration in X axis and then determine the value of Vmax and KM.3.
CONCLUSION
It is vital to have a good knowledge about the performance enzymes characteristics if they are to be utilized efficiently. The parameters Km and Vmax are therefore very important to determine.
REFERENCES
- Brigidi P, González-Vera A, Rossi M (1997). Study of stability of recombinant plasmids during the continuous culture of Bacillus stearothermophilus NUB3621 in nonselective medium. Biotechnol. Bioeng. 53: 507-514.
- Castro GR, Baigori MD, Sineriz F (1999). Studies on -amylase production by Bacillus licheniformis MIR-61. Acta Biotechnol. 19: 263-272.
- Bajpai P (1989) High temperature alkaline -amylase from Bacillus licheniformis TCRDC-B13. Biotech. Bioeng. 33: 72-78.
- Becker P, Abu-Resh I, Markossian S, Antranikian G, Mürkl H (1997). Determination of the kinetic parameters during continuous cultivation of the lipase-producing thermophile Bacillus sp. 1H1-91 on olive oil. Appl. Microbial. Biotechnol. 48: 184-190.
- Bernfeld P (1955). Amylases alpha and beta. Methods in enzymology. Academic Press, New York, pp. 140-146.
Also read:
- Estimation of protein by Folin Lowry method
- Isolation of Auxotrophic Antibiotic Resistant mutant by Induced mutagenesis in Bacteria by Replica plating technique
- Isolation of symbiotic N2-fixing bacteria from root nodules from leguminous plants
- Isolation and enumeration of phosphate solubilising bacteria from different habitats
- Determination of saponification value of the given oil/fat
- Determination of saponification value of fat (coconut oil)
- Protein denaturation by egg albumin method (in vitro)
🔴 Would you like to attempt Labmonk Daily quiz? Click here
🔵 Check out Jobs & Exam Notices. Labmonk Notice Board
🔴 Labmonk Scholarships. Click here
🔵 Labmonk Blog. Click here
🔴 Do you need notes? Click here
Watch Career related videos on Youtube: Watch now !!



