BACKGROUND
Aim:
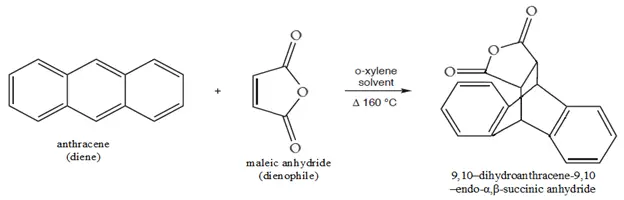
To synthesize 9, 10 – dihydroanthracene – 9, 10 – endo – α, β-succinic anhydride from anthracene (Diels-Alder reaction).
Principle:
The reaction of conjugated dienes with the ethylenic or acetylenic compound to form a six member cyclic product commonly known as “adduct” and reaction is known as Diels- Alder reaction. It is [4+2] cycloaddition involving reaction of diene (4π electrons) with dienophile (2π electrons) to form cyclic product.
This is an example of pericyclic or concerted reaction, where all the bond formations and bond breaking occur at the same time. In the same fashion anthracene (diene) reacts with maleic anhydride (dienophile) to give 9, 10-dihydroanthracene – 9, 10 – endo – α, β – succinic anhydride.1
Reaction:
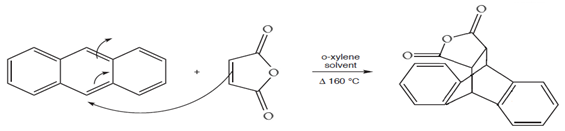
Mechanism:
Cyclo-addition reaction (Diels-Alder reaction) or Pericyclic reaction
REQUIREMENTS
Chemicals:
- Anthracene
- Maleic anhydride
- Xylene
- Activated charcoal
Apparatus:
- Beaker
- Pipette
- Round bottom flask – 250 ml
- Condenser
- Glass rod
- Buchner funnel
PROCEDURE
Transfer 4 g of anthracene, 2.2 g maleic anhydride and 50 ml of dry xylene in a 250 ml round bottom flask fitted with a reflux condenser. Boil the reaction mixture for about 30-35 min. under reflux and then allow it to cool down to room temperature.
If the reaction mixture appears to be colored, add 1 g of finely powdered activated charcoal and again reflux for 10-15 min. Filter the hot solution through Buchner funnel with suction and on subsequent cooling, in the filtrate colorless crystals of product (also known as adduct) are obtained.
The yield of the crude product is 4.3 g, having melting point 256-258 ͦ C. Recrystallize the crude product from about 50 ml of xylene, filter the hot solution through small preheated funnel, allow to cool the filtrate, so that solute soon crystalline out. Recrystallized product is obtained as colorless crystals, melting point 260-263 ͦ C, with yield of 4.1 g.
Calculation:
Here limiting reagent is anthracene; hence yield should be calculated from its amount taken.
C18H10 = Molecular formula of anthracene
C18H12O3 = Molecular formula of 9,10–dihydroanthracene-9,10–endo-α,β-succinic anhydride
Molecular weight of anthracene = 178 g/mole
Molecular weight of 9,10–dihydroanthracene-9,10–endo-α,β-succinic anhydride = 276 g/mole
Theoretical yield:
178 g anthracene forms 276 g product
Therefore, 4 g anthracene will form …….? (X) g product
X =( 276 ×4)/178 = 6.2 g
Theoretical yield = 6.2 g
Practical yield = ————- g
% Yield = (Practical Yield)/(Theoretical Yield) × 100
CONCLUSION
9,10–dihydroanthracene-9,10–endo-α,β-succinic anhydride was synthesized and the percentage yield was found to be………..%
REFERENCES
- Practical in organic chemistry, by Hitesh G. Raval, Sunil L. Baldania and Dimal A. Shah, Nirav Prakashan, Page no. 275.
Also read:
- Synthesis of 2-phenylindole from Phenyl Hydrazine
- Synthesis of anthranilic acid from phthalic anhydride
- Quantification of Danazol by ultraviolet spectrophotometer in capsules
- Calibration of ultraviolet spectrophotometer
- Calibration of pH meter
- Assay of Azithromycin by HPLC in tablet dosage form
- Assay of Cefadroxil by HPLC in tablet dosage form
- Preparation and standardization of sulfuric acid
- Determination of the concentration of potassium ion using Flame Photometry
🔴 Would you like to attempt Labmonk Daily quiz? Click here
🔵 Check out Jobs & Exam Notices. Labmonk Notice Board
🔴 Labmonk Scholarships. Click here
🔵 Labmonk Blog. Click here
🔴 Do you need notes? Click here



