[ps2id id=’background’ target=”/]
BACKGROUND
The leaves of plants contain a number of colored pigments generally falling into two categories, chlorophylls and carotenoids. Chlorophylls a and b are the pigments that make plants look green. Carotenoids are part of a larger collection of plant-derived compounds called terpenes. Carotenoids are tetraterpenes (eight isoprene units). Lycopene, the compound responsible for the red coloring of tomatoes and watermelon, and β -carotene, the compound that causes carrots and apricots to be orange, are examples of carotenoids. Spinach leaves contain chlorophyll a and b and β -carotene as major pigments as well as smaller amounts of other pigments.1 Chlorophyll a and chlorophyll b are similar in structure and may not be able to be resolved in this procedure.
[ps2id id=’requirements’ target=”/]
REQUIREMENTS
Chemicals: Petroleum ether (hexane) and acetone
Apparatus: Round bottom flask
Chromatography column
Pipettes
[ps2id id=’procedure’ target=”/]
PROCEDURE
Extraction of the pigments
About 5 grams of leaves is dried and placed in a mortar. Then the pigments are extracted by grinding the leaves with a pestle with about 5-10 ml of an 80:20 mixture (v/v), petroleum ether (hexane) and acetone. Then the liquid is decanted into a 50 ml round bottom flask. A quick filtration is done if necessary.
Preparation of the column
Wet pack method is used to prepare the column. The chromatography column is made with plastic tip with frit, the one-way stopcock, and the plastic funnel. The column is filled with enough alumina to get the required height. The dry alumina is poured into a beaker and hexane (pet ether) is added. The mixture is swirled and then poured into the column. The column is tapped gently, so air is not trapped as the alumina settles. Then it is added with a small amount of sand after the alumina has been settled. The column should not contain air bubbles and should be homogeneous. Then the solvent level is allowed to drop to the level of the alumina sand intersection.
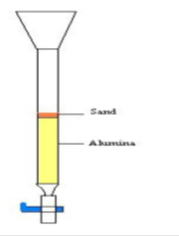
Running the column
Using a long pipette, some of the pigment mixture is added directly onto the sand. Then it is added enough to fill the sand layer with color. Then the stopcock is opened and let the liquid level falls to the top of the alumina. Gently added petroleum ether to fill the sand layer. Then the stopcock is opened and let the liquid level fall to the top of the alumina. These steps are repeated at least three times or until all the colored compounds are in the alumina. Now the column is filled with petroleum ether.
Do not ever let the column run dry. Never let the solvent level fall below the level of the alumina!
Then the stopcock is opened to allow a drip rate of approximately 1 drop per second. First the yellow-orange β-carotene is eluted. As the yellow-orange colored product is eluted, collected in a test tube. 3. When the β-carotene has been eluted, the elution of the chlorophylls are eluted by using a more polar solvent. Let the solvent level fall to the the top of the alumina. Gently filled the column with either pure acetone or a petroleum ether acetone combination. By using 70% petroleum ether: 30 % acetone combination, it might be able to separate chlorophylls a and b. Then the chlorophylls are collected in a separate test tube.
[ps2id id=’conclusion’ target=”/]
CONCLUSION
The Rf values of the above plant pigments are ___________.
[ps2id id=’references’ target=”/][ps2id id=’1′ target=”/]
REFERENCES
-
www.web.fscj.edu/milczanowski/ten/chloro.pdf.


